
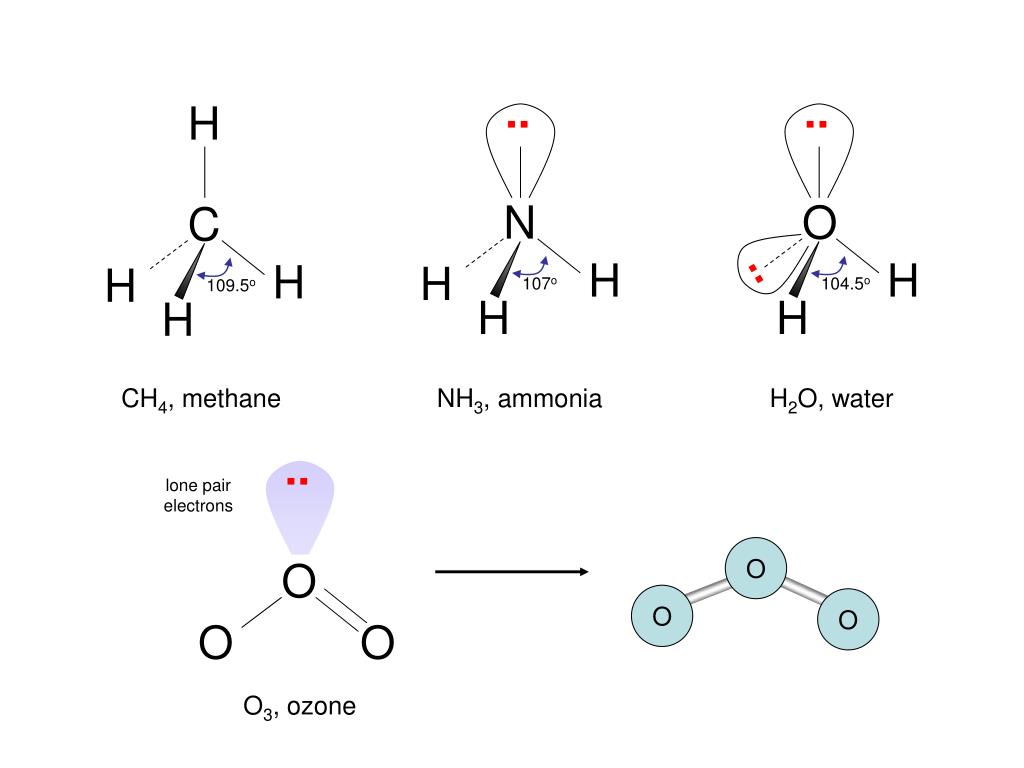

The net charge of the ozone molecule is zero because the positive and negative charges cancel out to each other. Ozone molecule contains an oxygen atom placed in the center containing a positive charge forming a double bond with one oxygen atom and a single bond with another negatively charged oxygen atom. Ozone is a triatomic molecule containing three oxygen atoms bound together by covalent bonds. In a Lewis structure, a dot represents a lone pair of electrons, whereas a straight line represents a bonded pair of electrons. The Lewis structure only considers the electrons in the valence shell of a molecule, ignoring the interior shells. The Lewis structure also shows the total number of lone and bound pairs of electrons in the atom. Lewis developed a diagrammatic method for representing a molecule, its chemical bonds, and a lone pair of electrons, known as the Lewis or electron dot structure. It is formed when UV light falls in diatomic oxygen under electrical discharges.ħ Electronegativity of O3 O 3 Lewis Structure.It is a strong oxidizing agent with highly reactive properties.It has a distinct pungent smell of chlorine.It is a pale blue inorganic gas with no taste.However, prolonged exposure to ozone for a long period of time causes fatal health hazards like lung cancer, chronic asthma, and circulatory diseases. Low concentration of ozone causes severe respiratory problems like chest pain, coughing, shortness of breath, and throat irritation.Īll these infections caused by exposure to ozone are acute but are reversible. If it comes in human contact it acts as a toxic gas.
O3 molecular geometry skin#
The high range UV light is not prevented by ozone but it does not cause severe actions on life and just causes mild sunburn and skin irritation.Īlthough ozone is the compound that makes life possible on earth, it does so by staying far away from human contact. The low range UV light is also prevented to enter by ozone but in a lesser amount. Ozone mainly prevents mid-range UV light from entering the earth. It blocks the harmful rays of the sun and does not allow them to reach the earth’s surroundings where life thrives. Ozone is formed when the UV light falls under diatomic oxygen in presence of electrical discharges in the Earth’s atmosphere. Ozone decomposes explosively at higher temperatures when present in high concentrations. It is both a natural and man-made chemical compound that is found abundantly in the earth’s upper atmosphere (stratosphere) and lower atmosphere (troposphere). At high concentrations, ozone is decayed into diatomic oxygen. Ozone is a very strong oxidizing agent and is highly unstable at higher concentrations. Ozone has a distinct pungent smell that is reminiscent of chlorine. Ozone or trioxygen (O 3) is a highly reactive inorganic gas made up of three oxygen atoms.


 0 kommentar(er)
0 kommentar(er)
